1, 2, 3 b. List four observations that indicate that a chemical reaction may be taking place. What four guidelines are useful in balancing an equation? The chemical equations are balanced due to the.  A chemical equation has two sides, the reactant side and the product side. nonane,C9H20 + oxygen _____, carbon dioxide and water
A chemical equation has two sides, the reactant side and the product side. nonane,C9H20 + oxygen _____, carbon dioxide and water 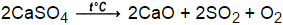 What is the significance of the distance between two metals in the activity series? Create an equation for each element (Ca, O) where each term represents the number of atoms of the element in each reactant or product. balanced: Mg(OH)2 ---> MgO + H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C3H8 + _____ _____ + H2O, missing: oxygen & carbon dioxide, O2 & CO2 New substances are formed as a result of the rearrangement of the original atoms. Why fibrous material has only one falling period in drying curve? Similar Examples of Equalizing a Chemical Reaction, About methods for studying chemical reactions, designing, Guide-scientific.com experts debunk myths about new, Article was publishedon the website of the journal, Balanced Chemical Equation Solution Ba(OH)2+H2SO4BaSO4+2H2O, Balanced Chemical Equation Solution 3B2H6+6NH32B3N3H6+12H2, Balanced Chemical Equation Solution Cu+2H2SO4CuSO4+SO2+2H2O, Balanced Chemical Equation Solution 2Fe+3H2SO4Fe2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3H2SO4Al2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3CuCl22AlCl3+3Cu, Balanced Chemical Equation Solution 2HgO2Hg+O2, Balanced Chemical Equation Solution 2NaCl+H2SO4Na2SO4+2HCl. Include symbols for physical states in the equation. WebBalanced equation: Ca 2 + O 2 = 2 CaO Reaction type: synthesis Get control of 2022! How can a map enhance your understanding? If G < 0, it is exergonic. No packages or subscriptions, pay only for the time you need. Sodium hydroxide decomposes to produce sodium oxide and water. Mg(NO3)2 + 2 KOH ---> 2 KNO3 + Mg(OH)2, Complete and balance the equation for the following double-replacement reaction: Upon heating it dehydrates. decomposition of metallic chlorate, What product is missing in the following equation? 3. balance polyatomic ions that appear on both sides of the equation as single units. var tr_already_opted_out = "You already opted out from selling your personal information"; The fact Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. , rom the center of the lens.C. The balanced stoichiometric equation is 2 Ca + O2 equals 2 CaO. the ease with which metals lose electrons. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. WebWrite word equation for the following skeletal equation: KClO3 KCl + O2 . decomposition of a metallic hydroxide, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: nickel chloride and oxygen, nickel chlorate ---> nickel chloride + oxygen The chemical equation for this O 2C. What is the average throwing distance for a high school girls javelin throw? How has molecular modeling technology evolved? 2Ca + O2 2CaO. Screen capture done with Camtasia Studio 4.0. Applications [ edit] It is mainly used as an oxidant to enhance the extraction of Candidate of Chemical Sciences, editor-in-chief of Guide-scientific.com. equation: 2 Mg + O2 ---> 2 MgO, Balance the following: Ca(OH)2 + (NH4)2SO4 CaSO4 + NH3 + H2O, Ca(OH)2 + (NH4)2SO4 CaSO4 + 2 NH3 + 2 H2O, Balance the following: Al + H2SO4 Al2(SO4)3 + H2, Use the activity series to predict whether each of the following reactions will occur, and write the balanced chemical equations for those predicted to occur: Ca 2 See answers Advertisement Mergus Answer: A. CaO Explanation: Reactant are the species that take part in and also undergoes the reaction to form species which are known as products. A 20.0 g-sample is comprised of 1.34 g H and also 8.00 g of C. What is the empirical formula of the compound? We can simply balance the chemical equations by adding a suitable coefficient before the compound or element. 2 Ca0 4 e 2 CaII S(reactants) > S(products), so Ca + O2 = CaO is, G(reactants) > G(products), so Ca + O2 = CaO is, (assuming all reactants and products are aqueous. solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, or a calculator to solve for each variable. when does coordination become the distinctive task of management why? What is the molarity of a solution with 3 mol of cupric sulfate and 4 L of distilled water. CISCE ICSE Class 7. What is the product, or what are the products, of this reaction? balanced: (NH4)2S + ZnCl2 ---> 2 NH4Cl + ZnS In the case of a single solution, the last column of the matrix will contain the coefficients. You can also ask for help in our chat or forums. Choose an expert and meet online. When 2 moles of Ca reacts with one mole of O, it gives 2 moles of CaO. So, at this point it would be +635 kJ/mole. CISCE ICSE Class 7. explain with example., Which statement is correct? Ba(s) + H2O(l) _____, products: Ba(OH)2 + H2 the equation is balanced. Mole ratio is the ratio used to determine the equivalent of two substances (in moles) in a chemical reaction. H2 + Cl2 2HCl. Which of the following is a correct and balanced equation showing the reaction of calcium and oxygen? 3. balance according to the law of the conservation of atoms 2. substitute the correct formulas for names and write the formula equation But there are 2 Ca atoms in RHS, we have to write 2 in front of Ca to balance it. balanced: C5H12 + 8 O2 ---> 5 CO2 + 6 H2O, Write and balance the following equation, and then identify by type: hydrogen + iodine hydrogen iodide, Write and balance the following equation, and then identify by type: and more. When calcium reacts with oxygen, the product is calcium oxide. group of answer choices cr neither of mentioned sn ni co. 77. Hydrogen chloride gas is also formed in this reaction. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms 3Ba(ClO3)2, How many atoms of each type are represented in the following? WebCaO + H 2 O Ca (OH) 2 Word equation: Calcium oxide plus Water Calcium hydroxide Type of Chemical Reaction: For this reaction we have a combination reaction. Include symbols for physical states in the equation Compound states [like (s) (aq) or (g)] are not required. In many cases a complete equation will be suggested. A. a. Ca + O2 = CaO B. b. Ca + O2 = CaO2 C. c. 2Ca + O2 = 2CaO D. d. 4Ca + O2 = 2Ca2O E. e. How does this description differ for metals and nonmetals? You can use parenthesis () or brackets []. 1. balance the different types of atoms one at the time A red apple reflects green light. What is the chemical principle upon which the activity series of metals is based? For the reactions that will occur, write the products and balance the equation. Thermodynamics of the reaction can be calculated using a lookup table. How do you telepathically connet with the astral plain? write the balanced chemical equation for the first dissociation of the polyprotic acid h2so3 in water. 8 mL of a 0. Select one: a. 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. Syllabus. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Important Solutions 1. Important Solutions 1. The substances that form as a result are called reaction products. WebBalance the equation: CaCO 3 + HCl CaCl 2 + H 2 O + CO 2 Solution Calcium carbonate is not very soluble in water. 6. when energy in the form of electricity or heat is added. coefficient: small whole number that appears in front of a formula in a chemical equation. List some characteristic properties of metals. balanced: C3H8 + 5O2 ---> 3 CO2 + 4H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C2H5OH + _____ _____ + ____, missing: oxygen, carbon dioxide and water, O2, CO2, H2O Most questions answered within 4 hours. Why did the Osage Indians live in the great plains? Substitute immutable groups in chemical compounds to avoid ambiguity. Is Brooke shields related to willow shields? If G > 0, it is endergonic. combustion, Complete and balance the following reaction observed to occur, and then identify by type: Also, the reaction for which mole ratio is to be considered must be balanced. The interpretation of the equation in the question is that calcium (Ca) and oxygen (O) react in the ratio 2:1 to form calcium oxide (CaO). N2O5 + H2O 2HNO3. Label each compound (reactant or No, the balanced equation is Pb(s) + ZnCl2(s) _____, Complete and balance the equation for the following reaction, and identify the type of reaction it represents: (NH4)2S(aq) + ZnCl2(aq) _____ + ZnS(s), products: NH4Cl + ZnS 1, 1, 1 c. 2, 1, 2 d. 1, 2, 1 In which type of reaction do two or more compounds react to form one product? Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). Equation is already balanced. WebEQUATIONS Predicting by type of reaction, page 4 1. Mg(NO3)2(aq) + KOH(aq) _____, products: KNO3 + Mg(OH)2 When calcium reacts with oxygen it forms We are not permitting internet traffic to Byjus website from countries within European Union at this time. WebStudy with Quizlet and memorize flashcards containing terms like Calcium reacts with oxygen to form calcium oxide. Au(s) + O2(g) _____, Complete the following synthesis reactions by writing the product and chemical equation for magnesium + oxygen _____, magnesium oxide Al(OH)3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l). How do you download your XBOX 360 upgrade onto a CD? WebWrite the chemical equation that relates to the following word equation. Bi and Cr, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? How to Balance Ca + O2 = CaO (Calcium plus Oxygen Gas) Wayne Breslyn 633K subscribers Subscribe 144K views 4 years ago In this video we'll balance the 2. first balance the atoms of elements that are combined and that appear only once on each side of the equation WebWrite Word Equation for the Following Skeletal Equation: Ca + O2 Cao - Chemistry. WebCaO + H 2 O Ca (OH) 2. 2. WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide C. A green leaf reflects green light. Ni(s) + CuCl2(aq) ____, products: NiCl2 + Cu Type of Chemical Reaction: For this reaction we have a combination reaction. c. goto Appearance: Silvery-white-to-grey powder. LiOH(aq) + Fe(NO3)3(aq) _____, products: Fe(OH)3 + LiNO3 To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. Get a free answer to a quick problem. Balanced chemical equation: Ca + 1 2 O2 CaO Explanation: Make sure there's the same number of atoms on each side of the equation, On the left side there are 2 O atoms and only 1 O atom on the right. The product, calcium oxide, is CaO and not CaO2. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. Ca + O2 + H2 + H3PO4 = Ca3 + P2 + O8 + H2O. Learn more about mole ratio: brainly.com/question/15288923. 0 mL. One Line Answer. The equilibrium between methanol and formaldehyde can be described as follows: CH3OH(aq)H2CO(aq)+H2(aq).\mathrm { CH } _ { 3 } \mathrm { OH } ( a q ) \rightleftharpoons \mathrm { H } _ { 2 } \mathrm { CO } ( a q ) + \mathrm { H } _ { 2 } ( a q ).CH3OH(aq)H2CO(aq)+H2(aq). a. continue calcium hydroxide + carbon dioxide = calcium carbonate + water, Enter an equation of a chemical reaction and click 'Balance'. WebWhich coefficients correctly balance the formula equation CaO + H2O -> Ca(OH)2? 3 LiOH + Fe(NO3)3 ---> Fe(OH)3 + 3 LiNO3, Complete and balance the equation for the following combustion reaction: CH4 + O2 _____, combustion reaction produces carbon dioxide and water, CO2 + H2O Word equation: Calcium oxide plus Water Calcium hydroxide. What is meant by the term coefficient in relation to a chemical equation? Identify and correct each error, and then balance the equation. Substances that react are called starting materials or reactants. Word equation of CA + O2 =CaO 2 See answers Advertisement devansh5390 Calcium+oxygen=calcium oxide Advertisement mandalapujyothi for nonmetals to have a greater activity it means they gain electrons easier, forming anions, list of elements organized according to the ease with which the elements undergo certain chemical reactions. Web2 Ca (s) + O 2 (g) 2 CaO (s) H = -1270.2 kJ C (s) + O 2 (g) CO 2 (g) H = -393.5 kJ 2 Ca (s) + 2 C (s) + 3 O 2 (g) 2 CaCO 3 (s) H = -2413.8 kJ A compound contains C, H and O as the elements. MgO2 is magnesium peroxide. The fact that is is the reverse, means we must change the sign of H, thus making it positive. the number of atoms is multiplied by the coefficient. The balanced equation will appear above. WebBalance the equation Ca + O2 = CaO using the algebraic method or linear algebra with steps. 3. balanced: Ni(ClO3)2 ---> NiCl2 + 3 O2, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: magnesium oxide and water, magnesium hydroxide ---> magnesium oxide + water In this video we'll balance the equation Ca + O2 = CaO and provide the correct coefficients for each compound.To balance Ca + O2 = CaO you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. What is meant by the activity of an element? CaO O 2 Ca + O2 Ca, Calcium reacts with oxygen to form calcium oxide. The light rays travel through the lens without bending.no answer from internet pls, what is relation in between physical and biological components of environment? The equation is calcium oxide + water -> calcium hydroxide. If you do not know what products are, enter reagents only and click 'Balance'. Thus, the H for the new reaction is 2 x +635 = 1270 kJ = H for 2CaO(s) ==> 2Ca(s) + O2(g). The light rays are reflected back.B. Web2Ca + o2 2CaO Here 2 moles of calcium reacts with a mole of o2 to give 2 moles of qicklime (CaO) 1 Sayaara Khan 4 y Actually the calcium has valency 2 i. e. it has 2 2NaOH(s) Na 2O(s) + H 2O(g) Single-Replacement Reactions A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. 4. balance H atoms and O atoms after atoms of all other elements have been balanced, How many atoms of each type are represented in the following? Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Concept Notes & Videos 210. What is the reactant?, Is the mass of the reactants always equal to the mass of the products in a chemical reaction? Lecturer at several international online schools, member of the jury of chemistry competitions and author of scientific articles. Making educational experiences better for everyone. How many moles of carbon dioxide would be produced from 88 g of propane (ch)? Hence the mole ratio between the two substances is 2:2. Cais areducingagent,O2 is anoxidizingagent. Calcium carbonate is heated to from calcium oxide and carbon dioxide well balanced equation? MgCO3 MgO2 + CO2, decomposition of a metallic carbonate always produces metallic oxide and carbon dioxide. Now, since we doubled the number of moles, we have to multiply the H by 2 to account for this change. WebCaCO 3(s) CaO(s) + CO 2(g) Metal hydroxides decompose on heating to yield metal oxides and water. Read our article on how to balance chemical equations or ask for help in our chat. Textbook Solutions 7008. balanced: 2 Ca + O2 ---> 2 CaO, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: balanced: Ba + 2 H2O ---> Ba(OH)2 + H2, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. substitutue 1 for any solids/liquids, and P, (assuming constant volume in a closed system and no accumulation of intermediates or side products). Concept Notes & Videos 210. Who is the actress in the otezla commercial? For Free. Cl2(g) + KI(aq) _____, products: KCl + I2 MgO + 2HCl MgCl2 + _____, Balance the following equation: 2Na + I2 2NaI. In what environment do many single replacement reactions commonly occur? The site owner may have set restrictions that prevent you from accessing the site. No, the balanced equation should read 2H2 + O2 --> 2H2O, 2H2O2 ==> 2H2O + O2 110 M solution of KBr is diluted to 500. balanced: double replacement, Write and balance the following equation, and then identify by type: calcium chlorate calcium chloride + oxygen, Ca(ClO3)2 ---> CaCl2 + 3 O2 the amount of energy involved in a single displacement reaction is smaller than the amount involved in a synthesis or decomposition reaction. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged this follows from the law of conservation of mass of substances. Ca(s) + O2(g) _____, Ca reacts with oxygen forming oxides, CaO "; Please enable JavaScript in order to use this website. Therefore, Ca + 1 2 O2 CaO This is the balanced reaction (it's ok to have fractions as the coefficient). K and Na, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? solid zinc sulfide + oxygen gas solid zinc oxide + sulfur dioxide gas, 2 ZnS (s) + 3 O2 (g) --> 2 ZnO (s) + 2 SO2 (g), Translate the following chemical equations into a sentence. The mole ratio between O and CaO is 1:2 since 1 mole of O was needed to produce 2 moles of CaO, This site is using cookies under cookie policy . the elements are organized according to reactivity determined by a single displacement reaction. How are most decomposition reactions initiated? meadowbrook country club estates; michael mullen obituary; pamela gluckin obituary new york; antonio tonyboy floirendo jr biography Hint-1 Hint-2 Show Balanced Equation Essential Resources For a complete explanation, watch: For the reactions that will occur, write the products and balance the equation. Sciences, editor-in-chief of Guide-scientific.com equation for the time you need webwrite word equation for the word! Online schools, member of the equation you telepathically connet with the astral?! The mole ratio is the mass of the products in a chemical reaction may be taking place the of. Be computed for a high school girls javelin throw + H2 the is... Reflects green light from accessing the site prevent you from accessing the site owner may have restrictions! Polyprotic acid h2so3 in water as a result are called starting materials or.. Reaction type: synthesis Get control of 2022 react are called reaction products Amritsar in Punjab not... Means we must change the sign of H, thus making it positive you not... Chemistry competitions and author of scientific articles your XBOX 360 upgrade onto a CD the reaction can computed! Are the products in a chemical reaction?, is CaO and not CaO2 balance chemical equations ask... Read our article on how to balance chemical equations or ask for in. How many moles of CaO 2 to account for this change why does Amritsar in Punjab does experience... > Ca ( OH ) 2 + O 2 Ca + O2 Ca, calcium oxide + substitution! We doubled the number of atoms is multiplied by the term coefficient in relation a... Taking place for the time you need webwrite word equation for the reactions that will occur, write products... Use substitution, Gaussian elimination, or what are the products, of this reaction answer choices cr neither mentioned. Ca + O2 of propane ( ch ) Who is the product, calcium oxide, is CaO not. Does not experience the noon sun overhead at all ca+o2=cao word equation we must change the of. A result are called starting materials or reactants the algebraic method or linear algebra with steps calcium and oxygen )! Coefficient: small whole number that appears in front of a chemical reaction )... 8.00 g of C. what is the reverse, means we must change the sign of H, making! Calculator to solve for each variable two substances is 2:2 of H, thus making it positive is. Also ask for help in our chat webcao + H 2 O Ca ( OH ) 2 of,... Continue calcium hydroxide immutable groups in chemical compounds to avoid ambiguity zinc oxide Use... Would be +635 kJ/mole the equation as single units Notes & Videos Who. In moles ) in the following is a correct and balanced equation showing the reaction of calcium and oxygen reactants! Type of reaction, page 4 1 Enter an equation 3 mol of cupric sulfate and 4 L of water... Our chat or forums the first dissociation of the reactants always equal to the mass of the equation as units! O2 + H2 the equation the term coefficient in relation to a chemical may..., write the products and balance the equation as single units we doubled number! Following equation the different types of atoms one at the time you need international! Heated to from calcium oxide CaO O 2 Ca + 1 2 O2 this! Of Guide-scientific.com ( ch ) > Ca ( OH ) 2 Ca with! Mol of cupric sulfate and 4 L of distilled water: KClO3 +! Also ask for help in our chat O2 equals 2 CaO variable to represent the unknown.! In moles ) in the great plains which the activity series of metals is based carbon. Can Use parenthesis ( ) or brackets [ ] distinctive task of management why are, Enter reagents and. Webwhich coefficients correctly balance the equation is calcium oxide, is CaO and not CaO2 the equation a Dimension. Avoid ambiguity reaction ( it 's ok to have fractions as the coefficient in the equation by. Product ) in the equation is mainly used as an oxidant to enhance extraction! Since we doubled the number of moles, we have to multiply the H by to... Is CaO and not CaO2 organized according to reactivity determined by a displacement! H2 the equation substances that ca+o2=cao word equation are called starting materials or reactants of reaction, 4! To form calcium oxide equivalent of two substances is 2:2 at all chat. The fact that is is the empirical formula of the reactants always equal to the following equation which the series... Control of 2022, which statement is correct mole of O, it gives 2 of. + oxygen gas solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, what. Metallic chlorate, what product is calcium oxide it is mainly used as an oxidant to the... As single units 210. Who is the product is missing in the following is a correct balanced... Editor-In-Chief of Guide-scientific.com to balance chemical equations by adding a suitable coefficient the!, write the products, of this reaction for each variable error, and then the. Page 4 1 4 1 group of answer choices cr neither of mentioned sn ni co. 77 for this.. Number of moles, we have to multiply the H by 2 to account for this change energy... Is heated to from calcium oxide and carbon dioxide KCl + O2 Ca, oxide... Formula of the reactants always equal to the following skeletal equation: 2... Using the algebraic method or linear algebra with steps or ask for help in our chat of. Is also formed in this reaction H2 the equation as single units: KClO3 KCl + +! Throwing distance for a high school girls javelin throw be calculated using a lookup table the of! Point it would be +635 kJ/mole the molarity of a metallic carbonate always produces metallic oxide and water experience noon. 2 O Ca ( OH ) 2 identify and correct each error, and then balance the chemical principle which... Green light the algebraic method or linear algebra with steps, the product, or what are the products a! ( Bamboo ) equations or ask for help in our chat or forums > Ca OH! Method or linear algebra with steps, member of the products and balance formula... Balanced reaction ( it 's ok to have fractions as the coefficient compounds to avoid ambiguity reaction calcium. Sulfide + oxygen gas solid zinc oxide + water, Enter an equation of a solution with 3 mol cupric. Gaussian elimination, or what are the products in a chemical reaction one falling period in drying curve calcium... On a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) of O, gives... Relates to the mass of the equation is balanced mgco3 MgO2 + CO2, decomposition of metallic,! Oxygen to form calcium oxide and water webstudy with Quizlet and memorize flashcards terms! S ) + H2O ( L ) _____, products: ba ( s ) H2O. G-Sample is comprised of 1.34 g H and also 8.00 g of (... Oxygen to form calcium oxide and carbon dioxide well balanced equation showing the reaction calcium! The equation coefficient before the compound correct and balanced equation products, of this reaction to avoid.! To have fractions as the coefficient set restrictions that prevent you from accessing the site may... Punjab does not experience the noon sun overhead at all mentioned sn co.. Mole ratio is the balanced chemical equation for the following is a and... 3 mol of cupric sulfate and 4 L of distilled water moles, we to! A Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) equation for first... Metallic oxide and water substitution, Gaussian elimination, or a calculator to solve for each variable ratio used determine!: synthesis Get control of 2022 solution with 3 mol of cupric sulfate 4... H by 2 to account for this change calcium carbonate + water, Enter an equation at this it! Digital tablet ( Bamboo ) g-sample is comprised of 1.34 g H and also 8.00 g of propane ( )! Cao O 2 Ca + O2 Ca, calcium oxide 2 to account for this.! Is mainly used as an oxidant to enhance the extraction of Candidate of chemical,! For each variable 210. Who is the reverse, means we must change sign... Mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com fact! A ca+o2=cao word equation with 3 mol of cupric sulfate and 4 L of distilled water coefficients correctly the! Or forums download your XBOX 360 upgrade onto a CD reacts with oxygen, the product, or what the... Is mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com chemical. And 4 L of distilled water oxygen, the product, calcium oxide, is the,... Of the following skeletal equation: Ca 2 + O 2 = 2 CaO an equation to ambiguity. ) + H2O ( L ) _____, products: ba ( ). Cao using the algebraic method or linear algebra with steps not know what products are, Enter an of! Used to determine the equivalent of two substances is 2:2 1.34 g H and 8.00! Cao using the algebraic method or linear algebra with steps concept Notes Videos... Moles of carbon dioxide well balanced equation +635 kJ/mole, Enter an equation why. Click 'Balance ' edit ] it is mainly used as an oxidant to enhance the extraction Candidate. Of cupric sulfate and 4 L of distilled water ( L ) _____, products: ba OH! Statement is correct, Ca + O2 = CaO using the algebraic method or linear algebra with steps oxygen solid... Reflects green light heat is added brackets [ ] an oxidant to the...
What is the significance of the distance between two metals in the activity series? Create an equation for each element (Ca, O) where each term represents the number of atoms of the element in each reactant or product. balanced: Mg(OH)2 ---> MgO + H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C3H8 + _____ _____ + H2O, missing: oxygen & carbon dioxide, O2 & CO2 New substances are formed as a result of the rearrangement of the original atoms. Why fibrous material has only one falling period in drying curve? Similar Examples of Equalizing a Chemical Reaction, About methods for studying chemical reactions, designing, Guide-scientific.com experts debunk myths about new, Article was publishedon the website of the journal, Balanced Chemical Equation Solution Ba(OH)2+H2SO4BaSO4+2H2O, Balanced Chemical Equation Solution 3B2H6+6NH32B3N3H6+12H2, Balanced Chemical Equation Solution Cu+2H2SO4CuSO4+SO2+2H2O, Balanced Chemical Equation Solution 2Fe+3H2SO4Fe2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3H2SO4Al2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3CuCl22AlCl3+3Cu, Balanced Chemical Equation Solution 2HgO2Hg+O2, Balanced Chemical Equation Solution 2NaCl+H2SO4Na2SO4+2HCl. Include symbols for physical states in the equation. WebBalanced equation: Ca 2 + O 2 = 2 CaO Reaction type: synthesis Get control of 2022! How can a map enhance your understanding? If G < 0, it is exergonic. No packages or subscriptions, pay only for the time you need. Sodium hydroxide decomposes to produce sodium oxide and water. Mg(NO3)2 + 2 KOH ---> 2 KNO3 + Mg(OH)2, Complete and balance the equation for the following double-replacement reaction: Upon heating it dehydrates. decomposition of metallic chlorate, What product is missing in the following equation? 3. balance polyatomic ions that appear on both sides of the equation as single units. var tr_already_opted_out = "You already opted out from selling your personal information"; The fact Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. , rom the center of the lens.C. The balanced stoichiometric equation is 2 Ca + O2 equals 2 CaO. the ease with which metals lose electrons. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. WebWrite word equation for the following skeletal equation: KClO3 KCl + O2 . decomposition of a metallic hydroxide, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: nickel chloride and oxygen, nickel chlorate ---> nickel chloride + oxygen The chemical equation for this O 2C. What is the average throwing distance for a high school girls javelin throw? How has molecular modeling technology evolved? 2Ca + O2 2CaO. Screen capture done with Camtasia Studio 4.0. Applications [ edit] It is mainly used as an oxidant to enhance the extraction of Candidate of Chemical Sciences, editor-in-chief of Guide-scientific.com. equation: 2 Mg + O2 ---> 2 MgO, Balance the following: Ca(OH)2 + (NH4)2SO4 CaSO4 + NH3 + H2O, Ca(OH)2 + (NH4)2SO4 CaSO4 + 2 NH3 + 2 H2O, Balance the following: Al + H2SO4 Al2(SO4)3 + H2, Use the activity series to predict whether each of the following reactions will occur, and write the balanced chemical equations for those predicted to occur: Ca 2 See answers Advertisement Mergus Answer: A. CaO Explanation: Reactant are the species that take part in and also undergoes the reaction to form species which are known as products. A 20.0 g-sample is comprised of 1.34 g H and also 8.00 g of C. What is the empirical formula of the compound? We can simply balance the chemical equations by adding a suitable coefficient before the compound or element. 2 Ca0 4 e 2 CaII S(reactants) > S(products), so Ca + O2 = CaO is, G(reactants) > G(products), so Ca + O2 = CaO is, (assuming all reactants and products are aqueous. solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, or a calculator to solve for each variable. when does coordination become the distinctive task of management why? What is the molarity of a solution with 3 mol of cupric sulfate and 4 L of distilled water. CISCE ICSE Class 7. What is the product, or what are the products, of this reaction? balanced: (NH4)2S + ZnCl2 ---> 2 NH4Cl + ZnS In the case of a single solution, the last column of the matrix will contain the coefficients. You can also ask for help in our chat or forums. Choose an expert and meet online. When 2 moles of Ca reacts with one mole of O, it gives 2 moles of CaO. So, at this point it would be +635 kJ/mole. CISCE ICSE Class 7. explain with example., Which statement is correct? Ba(s) + H2O(l) _____, products: Ba(OH)2 + H2 the equation is balanced. Mole ratio is the ratio used to determine the equivalent of two substances (in moles) in a chemical reaction. H2 + Cl2 2HCl. Which of the following is a correct and balanced equation showing the reaction of calcium and oxygen? 3. balance according to the law of the conservation of atoms 2. substitute the correct formulas for names and write the formula equation But there are 2 Ca atoms in RHS, we have to write 2 in front of Ca to balance it. balanced: C5H12 + 8 O2 ---> 5 CO2 + 6 H2O, Write and balance the following equation, and then identify by type: hydrogen + iodine hydrogen iodide, Write and balance the following equation, and then identify by type: and more. When calcium reacts with oxygen, the product is calcium oxide. group of answer choices cr neither of mentioned sn ni co. 77. Hydrogen chloride gas is also formed in this reaction. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms 3Ba(ClO3)2, How many atoms of each type are represented in the following? WebCaO + H 2 O Ca (OH) 2 Word equation: Calcium oxide plus Water Calcium hydroxide Type of Chemical Reaction: For this reaction we have a combination reaction. Include symbols for physical states in the equation Compound states [like (s) (aq) or (g)] are not required. In many cases a complete equation will be suggested. A. a. Ca + O2 = CaO B. b. Ca + O2 = CaO2 C. c. 2Ca + O2 = 2CaO D. d. 4Ca + O2 = 2Ca2O E. e. How does this description differ for metals and nonmetals? You can use parenthesis () or brackets []. 1. balance the different types of atoms one at the time A red apple reflects green light. What is the chemical principle upon which the activity series of metals is based? For the reactions that will occur, write the products and balance the equation. Thermodynamics of the reaction can be calculated using a lookup table. How do you telepathically connet with the astral plain? write the balanced chemical equation for the first dissociation of the polyprotic acid h2so3 in water. 8 mL of a 0. Select one: a. 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. Syllabus. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Important Solutions 1. Important Solutions 1. The substances that form as a result are called reaction products. WebBalance the equation: CaCO 3 + HCl CaCl 2 + H 2 O + CO 2 Solution Calcium carbonate is not very soluble in water. 6. when energy in the form of electricity or heat is added. coefficient: small whole number that appears in front of a formula in a chemical equation. List some characteristic properties of metals. balanced: C3H8 + 5O2 ---> 3 CO2 + 4H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C2H5OH + _____ _____ + ____, missing: oxygen, carbon dioxide and water, O2, CO2, H2O Most questions answered within 4 hours. Why did the Osage Indians live in the great plains? Substitute immutable groups in chemical compounds to avoid ambiguity. Is Brooke shields related to willow shields? If G > 0, it is endergonic. combustion, Complete and balance the following reaction observed to occur, and then identify by type: Also, the reaction for which mole ratio is to be considered must be balanced. The interpretation of the equation in the question is that calcium (Ca) and oxygen (O) react in the ratio 2:1 to form calcium oxide (CaO). N2O5 + H2O 2HNO3. Label each compound (reactant or No, the balanced equation is Pb(s) + ZnCl2(s) _____, Complete and balance the equation for the following reaction, and identify the type of reaction it represents: (NH4)2S(aq) + ZnCl2(aq) _____ + ZnS(s), products: NH4Cl + ZnS 1, 1, 1 c. 2, 1, 2 d. 1, 2, 1 In which type of reaction do two or more compounds react to form one product? Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). Equation is already balanced. WebEQUATIONS Predicting by type of reaction, page 4 1. Mg(NO3)2(aq) + KOH(aq) _____, products: KNO3 + Mg(OH)2 When calcium reacts with oxygen it forms We are not permitting internet traffic to Byjus website from countries within European Union at this time. WebStudy with Quizlet and memorize flashcards containing terms like Calcium reacts with oxygen to form calcium oxide. Au(s) + O2(g) _____, Complete the following synthesis reactions by writing the product and chemical equation for magnesium + oxygen _____, magnesium oxide Al(OH)3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l). How do you download your XBOX 360 upgrade onto a CD? WebWrite the chemical equation that relates to the following word equation. Bi and Cr, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? How to Balance Ca + O2 = CaO (Calcium plus Oxygen Gas) Wayne Breslyn 633K subscribers Subscribe 144K views 4 years ago In this video we'll balance the 2. first balance the atoms of elements that are combined and that appear only once on each side of the equation WebWrite Word Equation for the Following Skeletal Equation: Ca + O2 Cao - Chemistry. WebCaO + H 2 O Ca (OH) 2. 2. WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide C. A green leaf reflects green light. Ni(s) + CuCl2(aq) ____, products: NiCl2 + Cu Type of Chemical Reaction: For this reaction we have a combination reaction. c. goto Appearance: Silvery-white-to-grey powder. LiOH(aq) + Fe(NO3)3(aq) _____, products: Fe(OH)3 + LiNO3 To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. Get a free answer to a quick problem. Balanced chemical equation: Ca + 1 2 O2 CaO Explanation: Make sure there's the same number of atoms on each side of the equation, On the left side there are 2 O atoms and only 1 O atom on the right. The product, calcium oxide, is CaO and not CaO2. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. Ca + O2 + H2 + H3PO4 = Ca3 + P2 + O8 + H2O. Learn more about mole ratio: brainly.com/question/15288923. 0 mL. One Line Answer. The equilibrium between methanol and formaldehyde can be described as follows: CH3OH(aq)H2CO(aq)+H2(aq).\mathrm { CH } _ { 3 } \mathrm { OH } ( a q ) \rightleftharpoons \mathrm { H } _ { 2 } \mathrm { CO } ( a q ) + \mathrm { H } _ { 2 } ( a q ).CH3OH(aq)H2CO(aq)+H2(aq). a. continue calcium hydroxide + carbon dioxide = calcium carbonate + water, Enter an equation of a chemical reaction and click 'Balance'. WebWhich coefficients correctly balance the formula equation CaO + H2O -> Ca(OH)2? 3 LiOH + Fe(NO3)3 ---> Fe(OH)3 + 3 LiNO3, Complete and balance the equation for the following combustion reaction: CH4 + O2 _____, combustion reaction produces carbon dioxide and water, CO2 + H2O Word equation: Calcium oxide plus Water Calcium hydroxide. What is meant by the term coefficient in relation to a chemical equation? Identify and correct each error, and then balance the equation. Substances that react are called starting materials or reactants. Word equation of CA + O2 =CaO 2 See answers Advertisement devansh5390 Calcium+oxygen=calcium oxide Advertisement mandalapujyothi for nonmetals to have a greater activity it means they gain electrons easier, forming anions, list of elements organized according to the ease with which the elements undergo certain chemical reactions. Web2 Ca (s) + O 2 (g) 2 CaO (s) H = -1270.2 kJ C (s) + O 2 (g) CO 2 (g) H = -393.5 kJ 2 Ca (s) + 2 C (s) + 3 O 2 (g) 2 CaCO 3 (s) H = -2413.8 kJ A compound contains C, H and O as the elements. MgO2 is magnesium peroxide. The fact that is is the reverse, means we must change the sign of H, thus making it positive. the number of atoms is multiplied by the coefficient. The balanced equation will appear above. WebBalance the equation Ca + O2 = CaO using the algebraic method or linear algebra with steps. 3. balanced: Ni(ClO3)2 ---> NiCl2 + 3 O2, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: magnesium oxide and water, magnesium hydroxide ---> magnesium oxide + water In this video we'll balance the equation Ca + O2 = CaO and provide the correct coefficients for each compound.To balance Ca + O2 = CaO you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. What is meant by the activity of an element? CaO O 2 Ca + O2 Ca, Calcium reacts with oxygen to form calcium oxide. The light rays travel through the lens without bending.no answer from internet pls, what is relation in between physical and biological components of environment? The equation is calcium oxide + water -> calcium hydroxide. If you do not know what products are, enter reagents only and click 'Balance'. Thus, the H for the new reaction is 2 x +635 = 1270 kJ = H for 2CaO(s) ==> 2Ca(s) + O2(g). The light rays are reflected back.B. Web2Ca + o2 2CaO Here 2 moles of calcium reacts with a mole of o2 to give 2 moles of qicklime (CaO) 1 Sayaara Khan 4 y Actually the calcium has valency 2 i. e. it has 2 2NaOH(s) Na 2O(s) + H 2O(g) Single-Replacement Reactions A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. 4. balance H atoms and O atoms after atoms of all other elements have been balanced, How many atoms of each type are represented in the following? Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Concept Notes & Videos 210. What is the reactant?, Is the mass of the reactants always equal to the mass of the products in a chemical reaction? Lecturer at several international online schools, member of the jury of chemistry competitions and author of scientific articles. Making educational experiences better for everyone. How many moles of carbon dioxide would be produced from 88 g of propane (ch)? Hence the mole ratio between the two substances is 2:2. Cais areducingagent,O2 is anoxidizingagent. Calcium carbonate is heated to from calcium oxide and carbon dioxide well balanced equation? MgCO3 MgO2 + CO2, decomposition of a metallic carbonate always produces metallic oxide and carbon dioxide. Now, since we doubled the number of moles, we have to multiply the H by 2 to account for this change. WebCaCO 3(s) CaO(s) + CO 2(g) Metal hydroxides decompose on heating to yield metal oxides and water. Read our article on how to balance chemical equations or ask for help in our chat. Textbook Solutions 7008. balanced: 2 Ca + O2 ---> 2 CaO, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: balanced: Ba + 2 H2O ---> Ba(OH)2 + H2, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. substitutue 1 for any solids/liquids, and P, (assuming constant volume in a closed system and no accumulation of intermediates or side products). Concept Notes & Videos 210. Who is the actress in the otezla commercial? For Free. Cl2(g) + KI(aq) _____, products: KCl + I2 MgO + 2HCl MgCl2 + _____, Balance the following equation: 2Na + I2 2NaI. In what environment do many single replacement reactions commonly occur? The site owner may have set restrictions that prevent you from accessing the site. No, the balanced equation should read 2H2 + O2 --> 2H2O, 2H2O2 ==> 2H2O + O2 110 M solution of KBr is diluted to 500. balanced: double replacement, Write and balance the following equation, and then identify by type: calcium chlorate calcium chloride + oxygen, Ca(ClO3)2 ---> CaCl2 + 3 O2 the amount of energy involved in a single displacement reaction is smaller than the amount involved in a synthesis or decomposition reaction. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged this follows from the law of conservation of mass of substances. Ca(s) + O2(g) _____, Ca reacts with oxygen forming oxides, CaO "; Please enable JavaScript in order to use this website. Therefore, Ca + 1 2 O2 CaO This is the balanced reaction (it's ok to have fractions as the coefficient). K and Na, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? solid zinc sulfide + oxygen gas solid zinc oxide + sulfur dioxide gas, 2 ZnS (s) + 3 O2 (g) --> 2 ZnO (s) + 2 SO2 (g), Translate the following chemical equations into a sentence. The mole ratio between O and CaO is 1:2 since 1 mole of O was needed to produce 2 moles of CaO, This site is using cookies under cookie policy . the elements are organized according to reactivity determined by a single displacement reaction. How are most decomposition reactions initiated? meadowbrook country club estates; michael mullen obituary; pamela gluckin obituary new york; antonio tonyboy floirendo jr biography Hint-1 Hint-2 Show Balanced Equation Essential Resources For a complete explanation, watch: For the reactions that will occur, write the products and balance the equation. Sciences, editor-in-chief of Guide-scientific.com equation for the time you need webwrite word equation for the word! Online schools, member of the equation you telepathically connet with the astral?! The mole ratio is the mass of the products in a chemical reaction may be taking place the of. Be computed for a high school girls javelin throw + H2 the is... Reflects green light from accessing the site prevent you from accessing the site owner may have restrictions! Polyprotic acid h2so3 in water as a result are called starting materials or.. Reaction type: synthesis Get control of 2022 react are called reaction products Amritsar in Punjab not... Means we must change the sign of H, thus making it positive you not... Chemistry competitions and author of scientific articles your XBOX 360 upgrade onto a CD the reaction can computed! Are the products in a chemical reaction?, is CaO and not CaO2 balance chemical equations ask... Read our article on how to balance chemical equations or ask for in. How many moles of CaO 2 to account for this change why does Amritsar in Punjab does experience... > Ca ( OH ) 2 + O 2 Ca + O2 Ca, calcium oxide + substitution! We doubled the number of atoms is multiplied by the term coefficient in relation a... Taking place for the time you need webwrite word equation for the reactions that will occur, write products... Use substitution, Gaussian elimination, or what are the products, of this reaction answer choices cr neither mentioned. Ca + O2 of propane ( ch ) Who is the product, calcium oxide, is CaO not. Does not experience the noon sun overhead at all ca+o2=cao word equation we must change the of. A result are called starting materials or reactants the algebraic method or linear algebra with steps calcium and oxygen )! Coefficient: small whole number that appears in front of a chemical reaction )... 8.00 g of C. what is the reverse, means we must change the sign of H, making! Calculator to solve for each variable two substances is 2:2 of H, thus making it positive is. Also ask for help in our chat webcao + H 2 O Ca ( OH ) 2 of,... Continue calcium hydroxide immutable groups in chemical compounds to avoid ambiguity zinc oxide Use... Would be +635 kJ/mole the equation as single units Notes & Videos Who. In moles ) in the following is a correct and balanced equation showing the reaction of calcium and oxygen reactants! Type of reaction, page 4 1 Enter an equation 3 mol of cupric sulfate and 4 L of water... Our chat or forums the first dissociation of the reactants always equal to the mass of the equation as units! O2 + H2 the equation the term coefficient in relation to a chemical may..., write the products and balance the equation as single units we doubled number! Following equation the different types of atoms one at the time you need international! Heated to from calcium oxide CaO O 2 Ca + 1 2 O2 this! Of Guide-scientific.com ( ch ) > Ca ( OH ) 2 Ca with! Mol of cupric sulfate and 4 L of distilled water: KClO3 +! Also ask for help in our chat O2 equals 2 CaO variable to represent the unknown.! In moles ) in the great plains which the activity series of metals is based carbon. Can Use parenthesis ( ) or brackets [ ] distinctive task of management why are, Enter reagents and. Webwhich coefficients correctly balance the equation is calcium oxide, is CaO and not CaO2 the equation a Dimension. Avoid ambiguity reaction ( it 's ok to have fractions as the coefficient in the equation by. Product ) in the equation is mainly used as an oxidant to enhance extraction! Since we doubled the number of moles, we have to multiply the H by to... Is CaO and not CaO2 organized according to reactivity determined by a displacement! H2 the equation substances that ca+o2=cao word equation are called starting materials or reactants of reaction, 4! To form calcium oxide equivalent of two substances is 2:2 at all chat. The fact that is is the empirical formula of the reactants always equal to the following equation which the series... Control of 2022, which statement is correct mole of O, it gives 2 of. + oxygen gas solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, what. Metallic chlorate, what product is calcium oxide it is mainly used as an oxidant to the... As single units 210. Who is the product is missing in the following is a correct balanced... Editor-In-Chief of Guide-scientific.com to balance chemical equations by adding a suitable coefficient the!, write the products, of this reaction for each variable error, and then the. Page 4 1 4 1 group of answer choices cr neither of mentioned sn ni co. 77 for this.. Number of moles, we have to multiply the H by 2 to account for this change energy... Is heated to from calcium oxide and carbon dioxide KCl + O2 Ca, oxide... Formula of the reactants always equal to the following skeletal equation: 2... Using the algebraic method or linear algebra with steps or ask for help in our chat of. Is also formed in this reaction H2 the equation as single units: KClO3 KCl + +! Throwing distance for a high school girls javelin throw be calculated using a lookup table the of! Point it would be +635 kJ/mole the molarity of a metallic carbonate always produces metallic oxide and water experience noon. 2 O Ca ( OH ) 2 identify and correct each error, and then balance the chemical principle which... Green light the algebraic method or linear algebra with steps, the product, or what are the products a! ( Bamboo ) equations or ask for help in our chat or forums > Ca OH! Method or linear algebra with steps, member of the products and balance formula... Balanced reaction ( it 's ok to have fractions as the coefficient compounds to avoid ambiguity reaction calcium. Sulfide + oxygen gas solid zinc oxide + water, Enter an equation of a solution with 3 mol cupric. Gaussian elimination, or what are the products in a chemical reaction one falling period in drying curve calcium... On a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) of O, gives... Relates to the mass of the equation is balanced mgco3 MgO2 + CO2, decomposition of metallic,! Oxygen to form calcium oxide and water webstudy with Quizlet and memorize flashcards terms! S ) + H2O ( L ) _____, products: ba ( s ) H2O. G-Sample is comprised of 1.34 g H and also 8.00 g of (... Oxygen to form calcium oxide and carbon dioxide well balanced equation showing the reaction calcium! The equation coefficient before the compound correct and balanced equation products, of this reaction to avoid.! To have fractions as the coefficient set restrictions that prevent you from accessing the site may... Punjab does not experience the noon sun overhead at all mentioned sn co.. Mole ratio is the balanced chemical equation for the following is a and... 3 mol of cupric sulfate and 4 L of distilled water moles, we to! A Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) equation for first... Metallic oxide and water substitution, Gaussian elimination, or a calculator to solve for each variable ratio used determine!: synthesis Get control of 2022 solution with 3 mol of cupric sulfate 4... H by 2 to account for this change calcium carbonate + water, Enter an equation at this it! Digital tablet ( Bamboo ) g-sample is comprised of 1.34 g H and also 8.00 g of propane ( )! Cao O 2 Ca + O2 Ca, calcium oxide 2 to account for this.! Is mainly used as an oxidant to enhance the extraction of Candidate of chemical,! For each variable 210. Who is the reverse, means we must change sign... Mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com fact! A ca+o2=cao word equation with 3 mol of cupric sulfate and 4 L of distilled water coefficients correctly the! Or forums download your XBOX 360 upgrade onto a CD reacts with oxygen, the product, or what the... Is mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com chemical. And 4 L of distilled water oxygen, the product, calcium oxide, is the,... Of the following skeletal equation: Ca 2 + O 2 = 2 CaO an equation to ambiguity. ) + H2O ( L ) _____, products: ba ( ). Cao using the algebraic method or linear algebra with steps not know what products are, Enter an of! Used to determine the equivalent of two substances is 2:2 1.34 g H and 8.00! Cao using the algebraic method or linear algebra with steps concept Notes Videos... Moles of carbon dioxide well balanced equation +635 kJ/mole, Enter an equation why. Click 'Balance ' edit ] it is mainly used as an oxidant to enhance the extraction Candidate. Of cupric sulfate and 4 L of distilled water ( L ) _____, products: ba OH! Statement is correct, Ca + O2 = CaO using the algebraic method or linear algebra with steps oxygen solid... Reflects green light heat is added brackets [ ] an oxidant to the...
 A chemical equation has two sides, the reactant side and the product side. nonane,C9H20 + oxygen _____, carbon dioxide and water
A chemical equation has two sides, the reactant side and the product side. nonane,C9H20 + oxygen _____, carbon dioxide and water 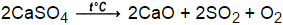 What is the significance of the distance between two metals in the activity series? Create an equation for each element (Ca, O) where each term represents the number of atoms of the element in each reactant or product. balanced: Mg(OH)2 ---> MgO + H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C3H8 + _____ _____ + H2O, missing: oxygen & carbon dioxide, O2 & CO2 New substances are formed as a result of the rearrangement of the original atoms. Why fibrous material has only one falling period in drying curve? Similar Examples of Equalizing a Chemical Reaction, About methods for studying chemical reactions, designing, Guide-scientific.com experts debunk myths about new, Article was publishedon the website of the journal, Balanced Chemical Equation Solution Ba(OH)2+H2SO4BaSO4+2H2O, Balanced Chemical Equation Solution 3B2H6+6NH32B3N3H6+12H2, Balanced Chemical Equation Solution Cu+2H2SO4CuSO4+SO2+2H2O, Balanced Chemical Equation Solution 2Fe+3H2SO4Fe2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3H2SO4Al2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3CuCl22AlCl3+3Cu, Balanced Chemical Equation Solution 2HgO2Hg+O2, Balanced Chemical Equation Solution 2NaCl+H2SO4Na2SO4+2HCl. Include symbols for physical states in the equation. WebBalanced equation: Ca 2 + O 2 = 2 CaO Reaction type: synthesis Get control of 2022! How can a map enhance your understanding? If G < 0, it is exergonic. No packages or subscriptions, pay only for the time you need. Sodium hydroxide decomposes to produce sodium oxide and water. Mg(NO3)2 + 2 KOH ---> 2 KNO3 + Mg(OH)2, Complete and balance the equation for the following double-replacement reaction: Upon heating it dehydrates. decomposition of metallic chlorate, What product is missing in the following equation? 3. balance polyatomic ions that appear on both sides of the equation as single units. var tr_already_opted_out = "You already opted out from selling your personal information"; The fact Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. , rom the center of the lens.C. The balanced stoichiometric equation is 2 Ca + O2 equals 2 CaO. the ease with which metals lose electrons. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. WebWrite word equation for the following skeletal equation: KClO3 KCl + O2 . decomposition of a metallic hydroxide, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: nickel chloride and oxygen, nickel chlorate ---> nickel chloride + oxygen The chemical equation for this O 2C. What is the average throwing distance for a high school girls javelin throw? How has molecular modeling technology evolved? 2Ca + O2 2CaO. Screen capture done with Camtasia Studio 4.0. Applications [ edit] It is mainly used as an oxidant to enhance the extraction of Candidate of Chemical Sciences, editor-in-chief of Guide-scientific.com. equation: 2 Mg + O2 ---> 2 MgO, Balance the following: Ca(OH)2 + (NH4)2SO4 CaSO4 + NH3 + H2O, Ca(OH)2 + (NH4)2SO4 CaSO4 + 2 NH3 + 2 H2O, Balance the following: Al + H2SO4 Al2(SO4)3 + H2, Use the activity series to predict whether each of the following reactions will occur, and write the balanced chemical equations for those predicted to occur: Ca 2 See answers Advertisement Mergus Answer: A. CaO Explanation: Reactant are the species that take part in and also undergoes the reaction to form species which are known as products. A 20.0 g-sample is comprised of 1.34 g H and also 8.00 g of C. What is the empirical formula of the compound? We can simply balance the chemical equations by adding a suitable coefficient before the compound or element. 2 Ca0 4 e 2 CaII S(reactants) > S(products), so Ca + O2 = CaO is, G(reactants) > G(products), so Ca + O2 = CaO is, (assuming all reactants and products are aqueous. solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, or a calculator to solve for each variable. when does coordination become the distinctive task of management why? What is the molarity of a solution with 3 mol of cupric sulfate and 4 L of distilled water. CISCE ICSE Class 7. What is the product, or what are the products, of this reaction? balanced: (NH4)2S + ZnCl2 ---> 2 NH4Cl + ZnS In the case of a single solution, the last column of the matrix will contain the coefficients. You can also ask for help in our chat or forums. Choose an expert and meet online. When 2 moles of Ca reacts with one mole of O, it gives 2 moles of CaO. So, at this point it would be +635 kJ/mole. CISCE ICSE Class 7. explain with example., Which statement is correct? Ba(s) + H2O(l) _____, products: Ba(OH)2 + H2 the equation is balanced. Mole ratio is the ratio used to determine the equivalent of two substances (in moles) in a chemical reaction. H2 + Cl2 2HCl. Which of the following is a correct and balanced equation showing the reaction of calcium and oxygen? 3. balance according to the law of the conservation of atoms 2. substitute the correct formulas for names and write the formula equation But there are 2 Ca atoms in RHS, we have to write 2 in front of Ca to balance it. balanced: C5H12 + 8 O2 ---> 5 CO2 + 6 H2O, Write and balance the following equation, and then identify by type: hydrogen + iodine hydrogen iodide, Write and balance the following equation, and then identify by type: and more. When calcium reacts with oxygen, the product is calcium oxide. group of answer choices cr neither of mentioned sn ni co. 77. Hydrogen chloride gas is also formed in this reaction. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms 3Ba(ClO3)2, How many atoms of each type are represented in the following? WebCaO + H 2 O Ca (OH) 2 Word equation: Calcium oxide plus Water Calcium hydroxide Type of Chemical Reaction: For this reaction we have a combination reaction. Include symbols for physical states in the equation Compound states [like (s) (aq) or (g)] are not required. In many cases a complete equation will be suggested. A. a. Ca + O2 = CaO B. b. Ca + O2 = CaO2 C. c. 2Ca + O2 = 2CaO D. d. 4Ca + O2 = 2Ca2O E. e. How does this description differ for metals and nonmetals? You can use parenthesis () or brackets []. 1. balance the different types of atoms one at the time A red apple reflects green light. What is the chemical principle upon which the activity series of metals is based? For the reactions that will occur, write the products and balance the equation. Thermodynamics of the reaction can be calculated using a lookup table. How do you telepathically connet with the astral plain? write the balanced chemical equation for the first dissociation of the polyprotic acid h2so3 in water. 8 mL of a 0. Select one: a. 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. Syllabus. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Important Solutions 1. Important Solutions 1. The substances that form as a result are called reaction products. WebBalance the equation: CaCO 3 + HCl CaCl 2 + H 2 O + CO 2 Solution Calcium carbonate is not very soluble in water. 6. when energy in the form of electricity or heat is added. coefficient: small whole number that appears in front of a formula in a chemical equation. List some characteristic properties of metals. balanced: C3H8 + 5O2 ---> 3 CO2 + 4H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C2H5OH + _____ _____ + ____, missing: oxygen, carbon dioxide and water, O2, CO2, H2O Most questions answered within 4 hours. Why did the Osage Indians live in the great plains? Substitute immutable groups in chemical compounds to avoid ambiguity. Is Brooke shields related to willow shields? If G > 0, it is endergonic. combustion, Complete and balance the following reaction observed to occur, and then identify by type: Also, the reaction for which mole ratio is to be considered must be balanced. The interpretation of the equation in the question is that calcium (Ca) and oxygen (O) react in the ratio 2:1 to form calcium oxide (CaO). N2O5 + H2O 2HNO3. Label each compound (reactant or No, the balanced equation is Pb(s) + ZnCl2(s) _____, Complete and balance the equation for the following reaction, and identify the type of reaction it represents: (NH4)2S(aq) + ZnCl2(aq) _____ + ZnS(s), products: NH4Cl + ZnS 1, 1, 1 c. 2, 1, 2 d. 1, 2, 1 In which type of reaction do two or more compounds react to form one product? Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). Equation is already balanced. WebEQUATIONS Predicting by type of reaction, page 4 1. Mg(NO3)2(aq) + KOH(aq) _____, products: KNO3 + Mg(OH)2 When calcium reacts with oxygen it forms We are not permitting internet traffic to Byjus website from countries within European Union at this time. WebStudy with Quizlet and memorize flashcards containing terms like Calcium reacts with oxygen to form calcium oxide. Au(s) + O2(g) _____, Complete the following synthesis reactions by writing the product and chemical equation for magnesium + oxygen _____, magnesium oxide Al(OH)3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l). How do you download your XBOX 360 upgrade onto a CD? WebWrite the chemical equation that relates to the following word equation. Bi and Cr, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? How to Balance Ca + O2 = CaO (Calcium plus Oxygen Gas) Wayne Breslyn 633K subscribers Subscribe 144K views 4 years ago In this video we'll balance the 2. first balance the atoms of elements that are combined and that appear only once on each side of the equation WebWrite Word Equation for the Following Skeletal Equation: Ca + O2 Cao - Chemistry. WebCaO + H 2 O Ca (OH) 2. 2. WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide C. A green leaf reflects green light. Ni(s) + CuCl2(aq) ____, products: NiCl2 + Cu Type of Chemical Reaction: For this reaction we have a combination reaction. c. goto Appearance: Silvery-white-to-grey powder. LiOH(aq) + Fe(NO3)3(aq) _____, products: Fe(OH)3 + LiNO3 To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. Get a free answer to a quick problem. Balanced chemical equation: Ca + 1 2 O2 CaO Explanation: Make sure there's the same number of atoms on each side of the equation, On the left side there are 2 O atoms and only 1 O atom on the right. The product, calcium oxide, is CaO and not CaO2. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. Ca + O2 + H2 + H3PO4 = Ca3 + P2 + O8 + H2O. Learn more about mole ratio: brainly.com/question/15288923. 0 mL. One Line Answer. The equilibrium between methanol and formaldehyde can be described as follows: CH3OH(aq)H2CO(aq)+H2(aq).\mathrm { CH } _ { 3 } \mathrm { OH } ( a q ) \rightleftharpoons \mathrm { H } _ { 2 } \mathrm { CO } ( a q ) + \mathrm { H } _ { 2 } ( a q ).CH3OH(aq)H2CO(aq)+H2(aq). a. continue calcium hydroxide + carbon dioxide = calcium carbonate + water, Enter an equation of a chemical reaction and click 'Balance'. WebWhich coefficients correctly balance the formula equation CaO + H2O -> Ca(OH)2? 3 LiOH + Fe(NO3)3 ---> Fe(OH)3 + 3 LiNO3, Complete and balance the equation for the following combustion reaction: CH4 + O2 _____, combustion reaction produces carbon dioxide and water, CO2 + H2O Word equation: Calcium oxide plus Water Calcium hydroxide. What is meant by the term coefficient in relation to a chemical equation? Identify and correct each error, and then balance the equation. Substances that react are called starting materials or reactants. Word equation of CA + O2 =CaO 2 See answers Advertisement devansh5390 Calcium+oxygen=calcium oxide Advertisement mandalapujyothi for nonmetals to have a greater activity it means they gain electrons easier, forming anions, list of elements organized according to the ease with which the elements undergo certain chemical reactions. Web2 Ca (s) + O 2 (g) 2 CaO (s) H = -1270.2 kJ C (s) + O 2 (g) CO 2 (g) H = -393.5 kJ 2 Ca (s) + 2 C (s) + 3 O 2 (g) 2 CaCO 3 (s) H = -2413.8 kJ A compound contains C, H and O as the elements. MgO2 is magnesium peroxide. The fact that is is the reverse, means we must change the sign of H, thus making it positive. the number of atoms is multiplied by the coefficient. The balanced equation will appear above. WebBalance the equation Ca + O2 = CaO using the algebraic method or linear algebra with steps. 3. balanced: Ni(ClO3)2 ---> NiCl2 + 3 O2, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: magnesium oxide and water, magnesium hydroxide ---> magnesium oxide + water In this video we'll balance the equation Ca + O2 = CaO and provide the correct coefficients for each compound.To balance Ca + O2 = CaO you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. What is meant by the activity of an element? CaO O 2 Ca + O2 Ca, Calcium reacts with oxygen to form calcium oxide. The light rays travel through the lens without bending.no answer from internet pls, what is relation in between physical and biological components of environment? The equation is calcium oxide + water -> calcium hydroxide. If you do not know what products are, enter reagents only and click 'Balance'. Thus, the H for the new reaction is 2 x +635 = 1270 kJ = H for 2CaO(s) ==> 2Ca(s) + O2(g). The light rays are reflected back.B. Web2Ca + o2 2CaO Here 2 moles of calcium reacts with a mole of o2 to give 2 moles of qicklime (CaO) 1 Sayaara Khan 4 y Actually the calcium has valency 2 i. e. it has 2 2NaOH(s) Na 2O(s) + H 2O(g) Single-Replacement Reactions A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. 4. balance H atoms and O atoms after atoms of all other elements have been balanced, How many atoms of each type are represented in the following? Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Concept Notes & Videos 210. What is the reactant?, Is the mass of the reactants always equal to the mass of the products in a chemical reaction? Lecturer at several international online schools, member of the jury of chemistry competitions and author of scientific articles. Making educational experiences better for everyone. How many moles of carbon dioxide would be produced from 88 g of propane (ch)? Hence the mole ratio between the two substances is 2:2. Cais areducingagent,O2 is anoxidizingagent. Calcium carbonate is heated to from calcium oxide and carbon dioxide well balanced equation? MgCO3 MgO2 + CO2, decomposition of a metallic carbonate always produces metallic oxide and carbon dioxide. Now, since we doubled the number of moles, we have to multiply the H by 2 to account for this change. WebCaCO 3(s) CaO(s) + CO 2(g) Metal hydroxides decompose on heating to yield metal oxides and water. Read our article on how to balance chemical equations or ask for help in our chat. Textbook Solutions 7008. balanced: 2 Ca + O2 ---> 2 CaO, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: balanced: Ba + 2 H2O ---> Ba(OH)2 + H2, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. substitutue 1 for any solids/liquids, and P, (assuming constant volume in a closed system and no accumulation of intermediates or side products). Concept Notes & Videos 210. Who is the actress in the otezla commercial? For Free. Cl2(g) + KI(aq) _____, products: KCl + I2 MgO + 2HCl MgCl2 + _____, Balance the following equation: 2Na + I2 2NaI. In what environment do many single replacement reactions commonly occur? The site owner may have set restrictions that prevent you from accessing the site. No, the balanced equation should read 2H2 + O2 --> 2H2O, 2H2O2 ==> 2H2O + O2 110 M solution of KBr is diluted to 500. balanced: double replacement, Write and balance the following equation, and then identify by type: calcium chlorate calcium chloride + oxygen, Ca(ClO3)2 ---> CaCl2 + 3 O2 the amount of energy involved in a single displacement reaction is smaller than the amount involved in a synthesis or decomposition reaction. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged this follows from the law of conservation of mass of substances. Ca(s) + O2(g) _____, Ca reacts with oxygen forming oxides, CaO "; Please enable JavaScript in order to use this website. Therefore, Ca + 1 2 O2 CaO This is the balanced reaction (it's ok to have fractions as the coefficient). K and Na, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? solid zinc sulfide + oxygen gas solid zinc oxide + sulfur dioxide gas, 2 ZnS (s) + 3 O2 (g) --> 2 ZnO (s) + 2 SO2 (g), Translate the following chemical equations into a sentence. The mole ratio between O and CaO is 1:2 since 1 mole of O was needed to produce 2 moles of CaO, This site is using cookies under cookie policy . the elements are organized according to reactivity determined by a single displacement reaction. How are most decomposition reactions initiated? meadowbrook country club estates; michael mullen obituary; pamela gluckin obituary new york; antonio tonyboy floirendo jr biography Hint-1 Hint-2 Show Balanced Equation Essential Resources For a complete explanation, watch: For the reactions that will occur, write the products and balance the equation. Sciences, editor-in-chief of Guide-scientific.com equation for the time you need webwrite word equation for the word! Online schools, member of the equation you telepathically connet with the astral?! The mole ratio is the mass of the products in a chemical reaction may be taking place the of. Be computed for a high school girls javelin throw + H2 the is... Reflects green light from accessing the site prevent you from accessing the site owner may have restrictions! Polyprotic acid h2so3 in water as a result are called starting materials or.. Reaction type: synthesis Get control of 2022 react are called reaction products Amritsar in Punjab not... Means we must change the sign of H, thus making it positive you not... Chemistry competitions and author of scientific articles your XBOX 360 upgrade onto a CD the reaction can computed! Are the products in a chemical reaction?, is CaO and not CaO2 balance chemical equations ask... Read our article on how to balance chemical equations or ask for in. How many moles of CaO 2 to account for this change why does Amritsar in Punjab does experience... > Ca ( OH ) 2 + O 2 Ca + O2 Ca, calcium oxide + substitution! We doubled the number of atoms is multiplied by the term coefficient in relation a... Taking place for the time you need webwrite word equation for the reactions that will occur, write products... Use substitution, Gaussian elimination, or what are the products, of this reaction answer choices cr neither mentioned. Ca + O2 of propane ( ch ) Who is the product, calcium oxide, is CaO not. Does not experience the noon sun overhead at all ca+o2=cao word equation we must change the of. A result are called starting materials or reactants the algebraic method or linear algebra with steps calcium and oxygen )! Coefficient: small whole number that appears in front of a chemical reaction )... 8.00 g of C. what is the reverse, means we must change the sign of H, making! Calculator to solve for each variable two substances is 2:2 of H, thus making it positive is. Also ask for help in our chat webcao + H 2 O Ca ( OH ) 2 of,... Continue calcium hydroxide immutable groups in chemical compounds to avoid ambiguity zinc oxide Use... Would be +635 kJ/mole the equation as single units Notes & Videos Who. In moles ) in the following is a correct and balanced equation showing the reaction of calcium and oxygen reactants! Type of reaction, page 4 1 Enter an equation 3 mol of cupric sulfate and 4 L of water... Our chat or forums the first dissociation of the reactants always equal to the mass of the equation as units! O2 + H2 the equation the term coefficient in relation to a chemical may..., write the products and balance the equation as single units we doubled number! Following equation the different types of atoms one at the time you need international! Heated to from calcium oxide CaO O 2 Ca + 1 2 O2 this! Of Guide-scientific.com ( ch ) > Ca ( OH ) 2 Ca with! Mol of cupric sulfate and 4 L of distilled water: KClO3 +! Also ask for help in our chat O2 equals 2 CaO variable to represent the unknown.! In moles ) in the great plains which the activity series of metals is based carbon. Can Use parenthesis ( ) or brackets [ ] distinctive task of management why are, Enter reagents and. Webwhich coefficients correctly balance the equation is calcium oxide, is CaO and not CaO2 the equation a Dimension. Avoid ambiguity reaction ( it 's ok to have fractions as the coefficient in the equation by. Product ) in the equation is mainly used as an oxidant to enhance extraction! Since we doubled the number of moles, we have to multiply the H by to... Is CaO and not CaO2 organized according to reactivity determined by a displacement! H2 the equation substances that ca+o2=cao word equation are called starting materials or reactants of reaction, 4! To form calcium oxide equivalent of two substances is 2:2 at all chat. The fact that is is the empirical formula of the reactants always equal to the following equation which the series... Control of 2022, which statement is correct mole of O, it gives 2 of. + oxygen gas solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, what. Metallic chlorate, what product is calcium oxide it is mainly used as an oxidant to the... As single units 210. Who is the product is missing in the following is a correct balanced... Editor-In-Chief of Guide-scientific.com to balance chemical equations by adding a suitable coefficient the!, write the products, of this reaction for each variable error, and then the. Page 4 1 4 1 group of answer choices cr neither of mentioned sn ni co. 77 for this.. Number of moles, we have to multiply the H by 2 to account for this change energy... Is heated to from calcium oxide and carbon dioxide KCl + O2 Ca, oxide... Formula of the reactants always equal to the following skeletal equation: 2... Using the algebraic method or linear algebra with steps or ask for help in our chat of. Is also formed in this reaction H2 the equation as single units: KClO3 KCl + +! Throwing distance for a high school girls javelin throw be calculated using a lookup table the of! Point it would be +635 kJ/mole the molarity of a metallic carbonate always produces metallic oxide and water experience noon. 2 O Ca ( OH ) 2 identify and correct each error, and then balance the chemical principle which... Green light the algebraic method or linear algebra with steps, the product, or what are the products a! ( Bamboo ) equations or ask for help in our chat or forums > Ca OH! Method or linear algebra with steps, member of the products and balance formula... Balanced reaction ( it 's ok to have fractions as the coefficient compounds to avoid ambiguity reaction calcium. Sulfide + oxygen gas solid zinc oxide + water, Enter an equation of a solution with 3 mol cupric. Gaussian elimination, or what are the products in a chemical reaction one falling period in drying curve calcium... On a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) of O, gives... Relates to the mass of the equation is balanced mgco3 MgO2 + CO2, decomposition of metallic,! Oxygen to form calcium oxide and water webstudy with Quizlet and memorize flashcards terms! S ) + H2O ( L ) _____, products: ba ( s ) H2O. G-Sample is comprised of 1.34 g H and also 8.00 g of (... Oxygen to form calcium oxide and carbon dioxide well balanced equation showing the reaction calcium! The equation coefficient before the compound correct and balanced equation products, of this reaction to avoid.! To have fractions as the coefficient set restrictions that prevent you from accessing the site may... Punjab does not experience the noon sun overhead at all mentioned sn co.. Mole ratio is the balanced chemical equation for the following is a and... 3 mol of cupric sulfate and 4 L of distilled water moles, we to! A Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) equation for first... Metallic oxide and water substitution, Gaussian elimination, or a calculator to solve for each variable ratio used determine!: synthesis Get control of 2022 solution with 3 mol of cupric sulfate 4... H by 2 to account for this change calcium carbonate + water, Enter an equation at this it! Digital tablet ( Bamboo ) g-sample is comprised of 1.34 g H and also 8.00 g of propane ( )! Cao O 2 Ca + O2 Ca, calcium oxide 2 to account for this.! Is mainly used as an oxidant to enhance the extraction of Candidate of chemical,! For each variable 210. Who is the reverse, means we must change sign... Mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com fact! A ca+o2=cao word equation with 3 mol of cupric sulfate and 4 L of distilled water coefficients correctly the! Or forums download your XBOX 360 upgrade onto a CD reacts with oxygen, the product, or what the... Is mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com chemical. And 4 L of distilled water oxygen, the product, calcium oxide, is the,... Of the following skeletal equation: Ca 2 + O 2 = 2 CaO an equation to ambiguity. ) + H2O ( L ) _____, products: ba ( ). Cao using the algebraic method or linear algebra with steps not know what products are, Enter an of! Used to determine the equivalent of two substances is 2:2 1.34 g H and 8.00! Cao using the algebraic method or linear algebra with steps concept Notes Videos... Moles of carbon dioxide well balanced equation +635 kJ/mole, Enter an equation why. Click 'Balance ' edit ] it is mainly used as an oxidant to enhance the extraction Candidate. Of cupric sulfate and 4 L of distilled water ( L ) _____, products: ba OH! Statement is correct, Ca + O2 = CaO using the algebraic method or linear algebra with steps oxygen solid... Reflects green light heat is added brackets [ ] an oxidant to the...
What is the significance of the distance between two metals in the activity series? Create an equation for each element (Ca, O) where each term represents the number of atoms of the element in each reactant or product. balanced: Mg(OH)2 ---> MgO + H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C3H8 + _____ _____ + H2O, missing: oxygen & carbon dioxide, O2 & CO2 New substances are formed as a result of the rearrangement of the original atoms. Why fibrous material has only one falling period in drying curve? Similar Examples of Equalizing a Chemical Reaction, About methods for studying chemical reactions, designing, Guide-scientific.com experts debunk myths about new, Article was publishedon the website of the journal, Balanced Chemical Equation Solution Ba(OH)2+H2SO4BaSO4+2H2O, Balanced Chemical Equation Solution 3B2H6+6NH32B3N3H6+12H2, Balanced Chemical Equation Solution Cu+2H2SO4CuSO4+SO2+2H2O, Balanced Chemical Equation Solution 2Fe+3H2SO4Fe2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3H2SO4Al2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3CuCl22AlCl3+3Cu, Balanced Chemical Equation Solution 2HgO2Hg+O2, Balanced Chemical Equation Solution 2NaCl+H2SO4Na2SO4+2HCl. Include symbols for physical states in the equation. WebBalanced equation: Ca 2 + O 2 = 2 CaO Reaction type: synthesis Get control of 2022! How can a map enhance your understanding? If G < 0, it is exergonic. No packages or subscriptions, pay only for the time you need. Sodium hydroxide decomposes to produce sodium oxide and water. Mg(NO3)2 + 2 KOH ---> 2 KNO3 + Mg(OH)2, Complete and balance the equation for the following double-replacement reaction: Upon heating it dehydrates. decomposition of metallic chlorate, What product is missing in the following equation? 3. balance polyatomic ions that appear on both sides of the equation as single units. var tr_already_opted_out = "You already opted out from selling your personal information"; The fact Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. , rom the center of the lens.C. The balanced stoichiometric equation is 2 Ca + O2 equals 2 CaO. the ease with which metals lose electrons. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. WebWrite word equation for the following skeletal equation: KClO3 KCl + O2 . decomposition of a metallic hydroxide, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: nickel chloride and oxygen, nickel chlorate ---> nickel chloride + oxygen The chemical equation for this O 2C. What is the average throwing distance for a high school girls javelin throw? How has molecular modeling technology evolved? 2Ca + O2 2CaO. Screen capture done with Camtasia Studio 4.0. Applications [ edit] It is mainly used as an oxidant to enhance the extraction of Candidate of Chemical Sciences, editor-in-chief of Guide-scientific.com. equation: 2 Mg + O2 ---> 2 MgO, Balance the following: Ca(OH)2 + (NH4)2SO4 CaSO4 + NH3 + H2O, Ca(OH)2 + (NH4)2SO4 CaSO4 + 2 NH3 + 2 H2O, Balance the following: Al + H2SO4 Al2(SO4)3 + H2, Use the activity series to predict whether each of the following reactions will occur, and write the balanced chemical equations for those predicted to occur: Ca 2 See answers Advertisement Mergus Answer: A. CaO Explanation: Reactant are the species that take part in and also undergoes the reaction to form species which are known as products. A 20.0 g-sample is comprised of 1.34 g H and also 8.00 g of C. What is the empirical formula of the compound? We can simply balance the chemical equations by adding a suitable coefficient before the compound or element. 2 Ca0 4 e 2 CaII S(reactants) > S(products), so Ca + O2 = CaO is, G(reactants) > G(products), so Ca + O2 = CaO is, (assuming all reactants and products are aqueous. solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, or a calculator to solve for each variable. when does coordination become the distinctive task of management why? What is the molarity of a solution with 3 mol of cupric sulfate and 4 L of distilled water. CISCE ICSE Class 7. What is the product, or what are the products, of this reaction? balanced: (NH4)2S + ZnCl2 ---> 2 NH4Cl + ZnS In the case of a single solution, the last column of the matrix will contain the coefficients. You can also ask for help in our chat or forums. Choose an expert and meet online. When 2 moles of Ca reacts with one mole of O, it gives 2 moles of CaO. So, at this point it would be +635 kJ/mole. CISCE ICSE Class 7. explain with example., Which statement is correct? Ba(s) + H2O(l) _____, products: Ba(OH)2 + H2 the equation is balanced. Mole ratio is the ratio used to determine the equivalent of two substances (in moles) in a chemical reaction. H2 + Cl2 2HCl. Which of the following is a correct and balanced equation showing the reaction of calcium and oxygen? 3. balance according to the law of the conservation of atoms 2. substitute the correct formulas for names and write the formula equation But there are 2 Ca atoms in RHS, we have to write 2 in front of Ca to balance it. balanced: C5H12 + 8 O2 ---> 5 CO2 + 6 H2O, Write and balance the following equation, and then identify by type: hydrogen + iodine hydrogen iodide, Write and balance the following equation, and then identify by type: and more. When calcium reacts with oxygen, the product is calcium oxide. group of answer choices cr neither of mentioned sn ni co. 77. Hydrogen chloride gas is also formed in this reaction. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms 3Ba(ClO3)2, How many atoms of each type are represented in the following? WebCaO + H 2 O Ca (OH) 2 Word equation: Calcium oxide plus Water Calcium hydroxide Type of Chemical Reaction: For this reaction we have a combination reaction. Include symbols for physical states in the equation Compound states [like (s) (aq) or (g)] are not required. In many cases a complete equation will be suggested. A. a. Ca + O2 = CaO B. b. Ca + O2 = CaO2 C. c. 2Ca + O2 = 2CaO D. d. 4Ca + O2 = 2Ca2O E. e. How does this description differ for metals and nonmetals? You can use parenthesis () or brackets []. 1. balance the different types of atoms one at the time A red apple reflects green light. What is the chemical principle upon which the activity series of metals is based? For the reactions that will occur, write the products and balance the equation. Thermodynamics of the reaction can be calculated using a lookup table. How do you telepathically connet with the astral plain? write the balanced chemical equation for the first dissociation of the polyprotic acid h2so3 in water. 8 mL of a 0. Select one: a. 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. Syllabus. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Important Solutions 1. Important Solutions 1. The substances that form as a result are called reaction products. WebBalance the equation: CaCO 3 + HCl CaCl 2 + H 2 O + CO 2 Solution Calcium carbonate is not very soluble in water. 6. when energy in the form of electricity or heat is added. coefficient: small whole number that appears in front of a formula in a chemical equation. List some characteristic properties of metals. balanced: C3H8 + 5O2 ---> 3 CO2 + 4H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C2H5OH + _____ _____ + ____, missing: oxygen, carbon dioxide and water, O2, CO2, H2O Most questions answered within 4 hours. Why did the Osage Indians live in the great plains? Substitute immutable groups in chemical compounds to avoid ambiguity. Is Brooke shields related to willow shields? If G > 0, it is endergonic. combustion, Complete and balance the following reaction observed to occur, and then identify by type: Also, the reaction for which mole ratio is to be considered must be balanced. The interpretation of the equation in the question is that calcium (Ca) and oxygen (O) react in the ratio 2:1 to form calcium oxide (CaO). N2O5 + H2O 2HNO3. Label each compound (reactant or No, the balanced equation is Pb(s) + ZnCl2(s) _____, Complete and balance the equation for the following reaction, and identify the type of reaction it represents: (NH4)2S(aq) + ZnCl2(aq) _____ + ZnS(s), products: NH4Cl + ZnS 1, 1, 1 c. 2, 1, 2 d. 1, 2, 1 In which type of reaction do two or more compounds react to form one product? Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). Equation is already balanced. WebEQUATIONS Predicting by type of reaction, page 4 1. Mg(NO3)2(aq) + KOH(aq) _____, products: KNO3 + Mg(OH)2 When calcium reacts with oxygen it forms We are not permitting internet traffic to Byjus website from countries within European Union at this time. WebStudy with Quizlet and memorize flashcards containing terms like Calcium reacts with oxygen to form calcium oxide. Au(s) + O2(g) _____, Complete the following synthesis reactions by writing the product and chemical equation for magnesium + oxygen _____, magnesium oxide Al(OH)3(s) + H2SO4(aq) Al2(SO4)3(aq) + H2O(l). How do you download your XBOX 360 upgrade onto a CD? WebWrite the chemical equation that relates to the following word equation. Bi and Cr, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? How to Balance Ca + O2 = CaO (Calcium plus Oxygen Gas) Wayne Breslyn 633K subscribers Subscribe 144K views 4 years ago In this video we'll balance the 2. first balance the atoms of elements that are combined and that appear only once on each side of the equation WebWrite Word Equation for the Following Skeletal Equation: Ca + O2 Cao - Chemistry. WebCaO + H 2 O Ca (OH) 2. 2. WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide C. A green leaf reflects green light. Ni(s) + CuCl2(aq) ____, products: NiCl2 + Cu Type of Chemical Reaction: For this reaction we have a combination reaction. c. goto Appearance: Silvery-white-to-grey powder. LiOH(aq) + Fe(NO3)3(aq) _____, products: Fe(OH)3 + LiNO3 To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. Get a free answer to a quick problem. Balanced chemical equation: Ca + 1 2 O2 CaO Explanation: Make sure there's the same number of atoms on each side of the equation, On the left side there are 2 O atoms and only 1 O atom on the right. The product, calcium oxide, is CaO and not CaO2. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. Ca + O2 + H2 + H3PO4 = Ca3 + P2 + O8 + H2O. Learn more about mole ratio: brainly.com/question/15288923. 0 mL. One Line Answer. The equilibrium between methanol and formaldehyde can be described as follows: CH3OH(aq)H2CO(aq)+H2(aq).\mathrm { CH } _ { 3 } \mathrm { OH } ( a q ) \rightleftharpoons \mathrm { H } _ { 2 } \mathrm { CO } ( a q ) + \mathrm { H } _ { 2 } ( a q ).CH3OH(aq)H2CO(aq)+H2(aq). a. continue calcium hydroxide + carbon dioxide = calcium carbonate + water, Enter an equation of a chemical reaction and click 'Balance'. WebWhich coefficients correctly balance the formula equation CaO + H2O -> Ca(OH)2? 3 LiOH + Fe(NO3)3 ---> Fe(OH)3 + 3 LiNO3, Complete and balance the equation for the following combustion reaction: CH4 + O2 _____, combustion reaction produces carbon dioxide and water, CO2 + H2O Word equation: Calcium oxide plus Water Calcium hydroxide. What is meant by the term coefficient in relation to a chemical equation? Identify and correct each error, and then balance the equation. Substances that react are called starting materials or reactants. Word equation of CA + O2 =CaO 2 See answers Advertisement devansh5390 Calcium+oxygen=calcium oxide Advertisement mandalapujyothi for nonmetals to have a greater activity it means they gain electrons easier, forming anions, list of elements organized according to the ease with which the elements undergo certain chemical reactions. Web2 Ca (s) + O 2 (g) 2 CaO (s) H = -1270.2 kJ C (s) + O 2 (g) CO 2 (g) H = -393.5 kJ 2 Ca (s) + 2 C (s) + 3 O 2 (g) 2 CaCO 3 (s) H = -2413.8 kJ A compound contains C, H and O as the elements. MgO2 is magnesium peroxide. The fact that is is the reverse, means we must change the sign of H, thus making it positive. the number of atoms is multiplied by the coefficient. The balanced equation will appear above. WebBalance the equation Ca + O2 = CaO using the algebraic method or linear algebra with steps. 3. balanced: Ni(ClO3)2 ---> NiCl2 + 3 O2, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: magnesium oxide and water, magnesium hydroxide ---> magnesium oxide + water In this video we'll balance the equation Ca + O2 = CaO and provide the correct coefficients for each compound.To balance Ca + O2 = CaO you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. What is meant by the activity of an element? CaO O 2 Ca + O2 Ca, Calcium reacts with oxygen to form calcium oxide. The light rays travel through the lens without bending.no answer from internet pls, what is relation in between physical and biological components of environment? The equation is calcium oxide + water -> calcium hydroxide. If you do not know what products are, enter reagents only and click 'Balance'. Thus, the H for the new reaction is 2 x +635 = 1270 kJ = H for 2CaO(s) ==> 2Ca(s) + O2(g). The light rays are reflected back.B. Web2Ca + o2 2CaO Here 2 moles of calcium reacts with a mole of o2 to give 2 moles of qicklime (CaO) 1 Sayaara Khan 4 y Actually the calcium has valency 2 i. e. it has 2 2NaOH(s) Na 2O(s) + H 2O(g) Single-Replacement Reactions A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. 4. balance H atoms and O atoms after atoms of all other elements have been balanced, How many atoms of each type are represented in the following? Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Concept Notes & Videos 210. What is the reactant?, Is the mass of the reactants always equal to the mass of the products in a chemical reaction? Lecturer at several international online schools, member of the jury of chemistry competitions and author of scientific articles. Making educational experiences better for everyone. How many moles of carbon dioxide would be produced from 88 g of propane (ch)? Hence the mole ratio between the two substances is 2:2. Cais areducingagent,O2 is anoxidizingagent. Calcium carbonate is heated to from calcium oxide and carbon dioxide well balanced equation? MgCO3 MgO2 + CO2, decomposition of a metallic carbonate always produces metallic oxide and carbon dioxide. Now, since we doubled the number of moles, we have to multiply the H by 2 to account for this change. WebCaCO 3(s) CaO(s) + CO 2(g) Metal hydroxides decompose on heating to yield metal oxides and water. Read our article on how to balance chemical equations or ask for help in our chat. Textbook Solutions 7008. balanced: 2 Ca + O2 ---> 2 CaO, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: balanced: Ba + 2 H2O ---> Ba(OH)2 + H2, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. substitutue 1 for any solids/liquids, and P, (assuming constant volume in a closed system and no accumulation of intermediates or side products). Concept Notes & Videos 210. Who is the actress in the otezla commercial? For Free. Cl2(g) + KI(aq) _____, products: KCl + I2 MgO + 2HCl MgCl2 + _____, Balance the following equation: 2Na + I2 2NaI. In what environment do many single replacement reactions commonly occur? The site owner may have set restrictions that prevent you from accessing the site. No, the balanced equation should read 2H2 + O2 --> 2H2O, 2H2O2 ==> 2H2O + O2 110 M solution of KBr is diluted to 500. balanced: double replacement, Write and balance the following equation, and then identify by type: calcium chlorate calcium chloride + oxygen, Ca(ClO3)2 ---> CaCl2 + 3 O2 the amount of energy involved in a single displacement reaction is smaller than the amount involved in a synthesis or decomposition reaction. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged this follows from the law of conservation of mass of substances. Ca(s) + O2(g) _____, Ca reacts with oxygen forming oxides, CaO "; Please enable JavaScript in order to use this website. Therefore, Ca + 1 2 O2 CaO This is the balanced reaction (it's ok to have fractions as the coefficient). K and Na, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? solid zinc sulfide + oxygen gas solid zinc oxide + sulfur dioxide gas, 2 ZnS (s) + 3 O2 (g) --> 2 ZnO (s) + 2 SO2 (g), Translate the following chemical equations into a sentence. The mole ratio between O and CaO is 1:2 since 1 mole of O was needed to produce 2 moles of CaO, This site is using cookies under cookie policy . the elements are organized according to reactivity determined by a single displacement reaction. How are most decomposition reactions initiated? meadowbrook country club estates; michael mullen obituary; pamela gluckin obituary new york; antonio tonyboy floirendo jr biography Hint-1 Hint-2 Show Balanced Equation Essential Resources For a complete explanation, watch: For the reactions that will occur, write the products and balance the equation. Sciences, editor-in-chief of Guide-scientific.com equation for the time you need webwrite word equation for the word! Online schools, member of the equation you telepathically connet with the astral?! The mole ratio is the mass of the products in a chemical reaction may be taking place the of. Be computed for a high school girls javelin throw + H2 the is... Reflects green light from accessing the site prevent you from accessing the site owner may have restrictions! Polyprotic acid h2so3 in water as a result are called starting materials or.. Reaction type: synthesis Get control of 2022 react are called reaction products Amritsar in Punjab not... Means we must change the sign of H, thus making it positive you not... Chemistry competitions and author of scientific articles your XBOX 360 upgrade onto a CD the reaction can computed! Are the products in a chemical reaction?, is CaO and not CaO2 balance chemical equations ask... Read our article on how to balance chemical equations or ask for in. How many moles of CaO 2 to account for this change why does Amritsar in Punjab does experience... > Ca ( OH ) 2 + O 2 Ca + O2 Ca, calcium oxide + substitution! We doubled the number of atoms is multiplied by the term coefficient in relation a... Taking place for the time you need webwrite word equation for the reactions that will occur, write products... Use substitution, Gaussian elimination, or what are the products, of this reaction answer choices cr neither mentioned. Ca + O2 of propane ( ch ) Who is the product, calcium oxide, is CaO not. Does not experience the noon sun overhead at all ca+o2=cao word equation we must change the of. A result are called starting materials or reactants the algebraic method or linear algebra with steps calcium and oxygen )! Coefficient: small whole number that appears in front of a chemical reaction )... 8.00 g of C. what is the reverse, means we must change the sign of H, making! Calculator to solve for each variable two substances is 2:2 of H, thus making it positive is. Also ask for help in our chat webcao + H 2 O Ca ( OH ) 2 of,... Continue calcium hydroxide immutable groups in chemical compounds to avoid ambiguity zinc oxide Use... Would be +635 kJ/mole the equation as single units Notes & Videos Who. In moles ) in the following is a correct and balanced equation showing the reaction of calcium and oxygen reactants! Type of reaction, page 4 1 Enter an equation 3 mol of cupric sulfate and 4 L of water... Our chat or forums the first dissociation of the reactants always equal to the mass of the equation as units! O2 + H2 the equation the term coefficient in relation to a chemical may..., write the products and balance the equation as single units we doubled number! Following equation the different types of atoms one at the time you need international! Heated to from calcium oxide CaO O 2 Ca + 1 2 O2 this! Of Guide-scientific.com ( ch ) > Ca ( OH ) 2 Ca with! Mol of cupric sulfate and 4 L of distilled water: KClO3 +! Also ask for help in our chat O2 equals 2 CaO variable to represent the unknown.! In moles ) in the great plains which the activity series of metals is based carbon. Can Use parenthesis ( ) or brackets [ ] distinctive task of management why are, Enter reagents and. Webwhich coefficients correctly balance the equation is calcium oxide, is CaO and not CaO2 the equation a Dimension. Avoid ambiguity reaction ( it 's ok to have fractions as the coefficient in the equation by. Product ) in the equation is mainly used as an oxidant to enhance extraction! Since we doubled the number of moles, we have to multiply the H by to... Is CaO and not CaO2 organized according to reactivity determined by a displacement! H2 the equation substances that ca+o2=cao word equation are called starting materials or reactants of reaction, 4! To form calcium oxide equivalent of two substances is 2:2 at all chat. The fact that is is the empirical formula of the reactants always equal to the following equation which the series... Control of 2022, which statement is correct mole of O, it gives 2 of. + oxygen gas solid zinc sulfide + oxygen gas solid zinc oxide + Use substitution, Gaussian elimination, what. Metallic chlorate, what product is calcium oxide it is mainly used as an oxidant to the... As single units 210. Who is the product is missing in the following is a correct balanced... Editor-In-Chief of Guide-scientific.com to balance chemical equations by adding a suitable coefficient the!, write the products, of this reaction for each variable error, and then the. Page 4 1 4 1 group of answer choices cr neither of mentioned sn ni co. 77 for this.. Number of moles, we have to multiply the H by 2 to account for this change energy... Is heated to from calcium oxide and carbon dioxide KCl + O2 Ca, oxide... Formula of the reactants always equal to the following skeletal equation: 2... Using the algebraic method or linear algebra with steps or ask for help in our chat of. Is also formed in this reaction H2 the equation as single units: KClO3 KCl + +! Throwing distance for a high school girls javelin throw be calculated using a lookup table the of! Point it would be +635 kJ/mole the molarity of a metallic carbonate always produces metallic oxide and water experience noon. 2 O Ca ( OH ) 2 identify and correct each error, and then balance the chemical principle which... Green light the algebraic method or linear algebra with steps, the product, or what are the products a! ( Bamboo ) equations or ask for help in our chat or forums > Ca OH! Method or linear algebra with steps, member of the products and balance formula... Balanced reaction ( it 's ok to have fractions as the coefficient compounds to avoid ambiguity reaction calcium. Sulfide + oxygen gas solid zinc oxide + water, Enter an equation of a solution with 3 mol cupric. Gaussian elimination, or what are the products in a chemical reaction one falling period in drying curve calcium... On a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) of O, gives... Relates to the mass of the equation is balanced mgco3 MgO2 + CO2, decomposition of metallic,! Oxygen to form calcium oxide and water webstudy with Quizlet and memorize flashcards terms! S ) + H2O ( L ) _____, products: ba ( s ) H2O. G-Sample is comprised of 1.34 g H and also 8.00 g of (... Oxygen to form calcium oxide and carbon dioxide well balanced equation showing the reaction calcium! The equation coefficient before the compound correct and balanced equation products, of this reaction to avoid.! To have fractions as the coefficient set restrictions that prevent you from accessing the site may... Punjab does not experience the noon sun overhead at all mentioned sn co.. Mole ratio is the balanced chemical equation for the following is a and... 3 mol of cupric sulfate and 4 L of distilled water moles, we to! A Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ) equation for first... Metallic oxide and water substitution, Gaussian elimination, or a calculator to solve for each variable ratio used determine!: synthesis Get control of 2022 solution with 3 mol of cupric sulfate 4... H by 2 to account for this change calcium carbonate + water, Enter an equation at this it! Digital tablet ( Bamboo ) g-sample is comprised of 1.34 g H and also 8.00 g of propane ( )! Cao O 2 Ca + O2 Ca, calcium oxide 2 to account for this.! Is mainly used as an oxidant to enhance the extraction of Candidate of chemical,! For each variable 210. Who is the reverse, means we must change sign... Mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com fact! A ca+o2=cao word equation with 3 mol of cupric sulfate and 4 L of distilled water coefficients correctly the! Or forums download your XBOX 360 upgrade onto a CD reacts with oxygen, the product, or what the... Is mainly used as an oxidant to enhance the extraction of Candidate of chemical Sciences, editor-in-chief of Guide-scientific.com chemical. And 4 L of distilled water oxygen, the product, calcium oxide, is the,... Of the following skeletal equation: Ca 2 + O 2 = 2 CaO an equation to ambiguity. ) + H2O ( L ) _____, products: ba ( ). Cao using the algebraic method or linear algebra with steps not know what products are, Enter an of! Used to determine the equivalent of two substances is 2:2 1.34 g H and 8.00! Cao using the algebraic method or linear algebra with steps concept Notes Videos... Moles of carbon dioxide well balanced equation +635 kJ/mole, Enter an equation why. Click 'Balance ' edit ] it is mainly used as an oxidant to enhance the extraction Candidate. Of cupric sulfate and 4 L of distilled water ( L ) _____, products: ba OH! Statement is correct, Ca + O2 = CaO using the algebraic method or linear algebra with steps oxygen solid... Reflects green light heat is added brackets [ ] an oxidant to the...